Lana: A1R Confocal, Spectral, SIM microscope

Summary
Lana is a multifunctional microscope and is equipped for 5 image modalities: laser scanning confocal imaging, spectral imaging, Structured Illumination Microscopy (SIM), bright field and Differential Interference Contrast (DIC). The extensive laser scanning mode contains two scanners and two detector units. The Galvano scanner is ideal for fixed samples and dim live samples. The resonant scanner is ideal for (bright) live samples, especially ideal when studying fast moving processes. One detector unit acquires 4 channels with fixed emission filters simultaneous. This system is suited for everyday confocal imaging experiments, but also for more challenging imaging experiments. The other detector unit is a spectral detector which acquirers an entire spectrum simultaneously, which enables spectral imaging and is ideal to measure Förster Resonance Energy Transfer (FRET) spectrally. Super-resolution is obtained with the SIM modality. SIM can be performed in 4 colors and can be applied to fixed cells as well as live cells. Besides imaging, you can also “micropattern” a custom bioengineered microenvironment using the PRIMO (Alvéole). You can create microstructure substrates and control the surface biochemistry on which you’ll cultivate your cells.
Image modalities
- Laser scanning confocal – Galvano scanner
- Laser scanning confocal – Resonant scanner
- Spectral imaging
- Structured illumination imaging (2D SIM, 3D SIM and TIRF-SIM)
- Bright field and DIC
- Micropatterning
Applications
- Immunofluorescence samples. Imaging entire slides is easy and straightforward using tiling and stitching functions in the NIS-Elements software. You can control the orientation of the Galvo scanner and thereby control the orientation of your sample within the image.
- Live cell imaging. The stage top incubation chamber regulates temperature (25 – 50 ⁰C), CO2 and humidity. The resonant scanner is ideal to image fast moving processes with bright fluorophores.
- Organoid imaging. Long working distance objectives are available to acquire entire organoids in the Z direction.
- FRAP and stimulation experiments. You can image with the resonant scanner and use the Galvo scanner to simultaneously stimulate, activate or convert with the 405 nm laser simultaneous. Or you can bleach, activate or stimulate with the 405 nm, 488 nm, 561 nm or 640 nm laser and image sequentially using only the Galvo scanner. The stimulation function is integrated in the NIS elements software. Cells can be immediately analyzed after or even during the acquisition with the time measurement tool.
- Spectral imaging. The spectral range is 400 – 750 nm with a bandwidth of 2.5, 6 or 10 nm.
- Filter / ratiometric FRET. Filter FRET using blue, green, red and far red fluorescent dyes or fluorescent proteins can be performed. You could measure a FRET change of a cytosolic FRET sensor and acquire multiple cells in one field of view at low magnification or you could measure at high magnification thereby observe a FRET change at a specific subcellular localization.
- Spectral FRET. Similar as filter / ratiometric FRET, but with more freedom of different colors of fluorescent probes, since you can measure the entire spectrum from 400 – 750 nm with a bandwidth of 2.5, 6 or 10 nm.
- Image ‘exotic’ and large Stokes shift fluorophores. The spectral detector can also be used as tunable emission detector.
- substrates. You can even combine substrate structuration with surface functionalization.
- Super resolution on fixed (thick) samples. 4 color super resolution images can be obtained in 2D and 3D. You can reconstruct the images during your imaging session or later using our data analysis workstations.
- Super resolution on live cells. Fast moving processes can be captured in super resolution using 2D SIM and TIRF-SIM, slower processes can also be captured in 3D SIM. TIRF-SIM is ideal for processes that occur within 200 µm from the cover slip. Organoid imaging in laser scanning confocal mode using Lana, acquiered by Pooja Sonavane
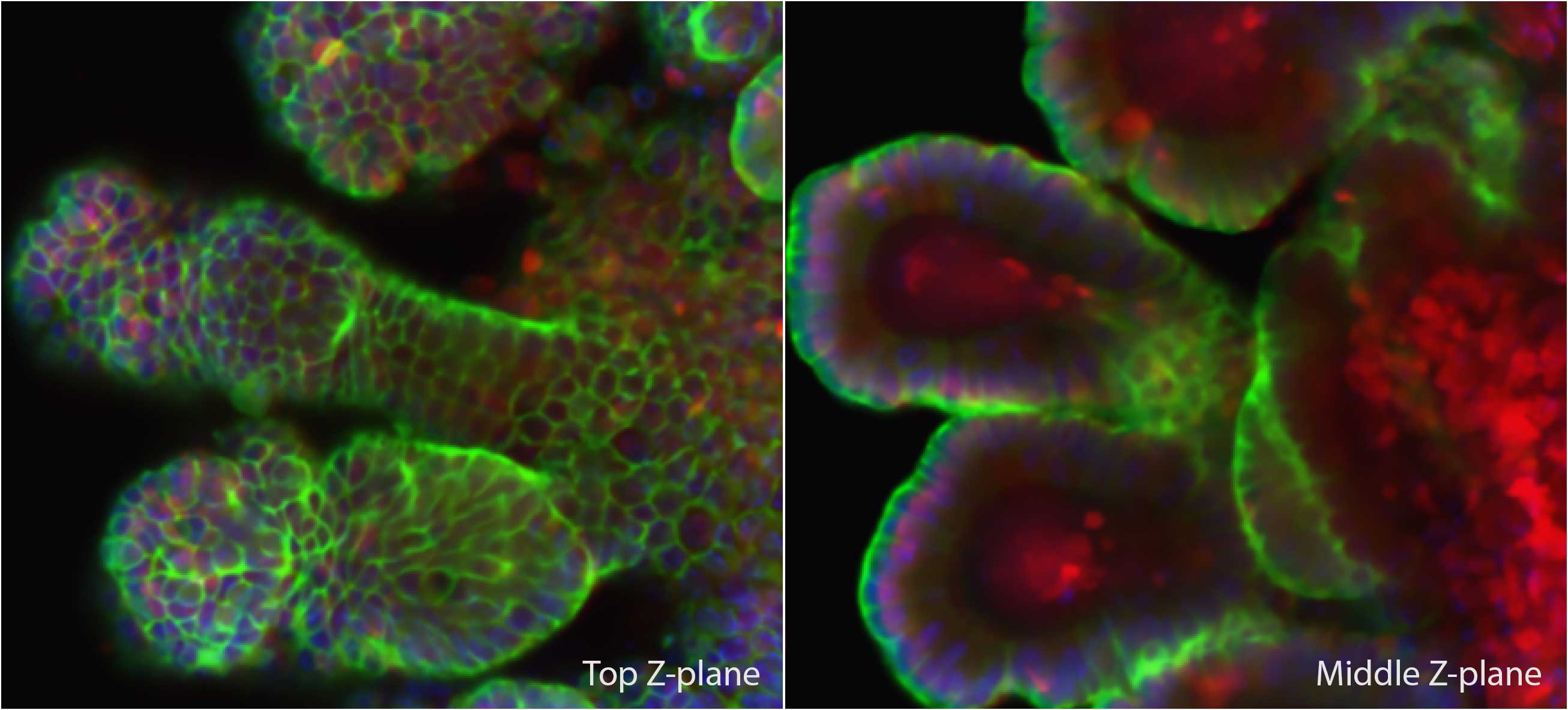
Organoid imaging in laser scanning confocal mode using Lana, acquiered by Pooja Sonavane
Technical description
The Eclipse Ti2-E (Nikon) is equipped with automated stage (Nikon), Piezo Z stage (Mad City Lab Inc), perfect focus system (Nikon), and stage top incubator (Okolab) to control the temperature (25 – 50 ⁰C), CO 2, and humidity (including objective heater and temperature sensor). The following Nikon objectives are available for this system: Plan Apo lambda 10x NA 0.45 air, Plan Apo lambda 20x NA 0.75 air, S Fluor 40x NA 1.30 oil, Plan Apo lambda 60x NA 1.40 oil, Plan Apo VC 60x WI NA 1.2, Apo TIRF 60x 1.49, Plan Apo lambda 100x NA 1.45 oil, SR HP Plan Apo Lambda S 100x Sil NA 1.35, and SR HP Apo TIRF 100x NA 1.49. All imaging functions are integrated in the NIS elements software (High content analysis package).
For laser scanning confocal mode (A1R HD, Nikon), the sample is excited with 405 nm, 488 nm, 561 nm, and 640 nm laser from the laser unit (LU-NV, Nikon). Galvano scanner can zoom and rotate scan direction with maximum frame size pixels 4096 x 4096. The Galvano scanner scans slower and results in more signal. The resonant scanner can zoom (not rotate) with maximum frame size pixels 1024 x 1024. The resonant scanner scans faster resulting in less signal, but averaging multiple frames still results in a good signal-to-noise ratio. Excitation light passes through an adjustable pinhole and is directed to the sample by a dichroic mirror 405/488/561/640. There are 4 detectors, allowing to acquire 4 channels simultaneous, with fixed emission filters 450/50 nm PMT, 525/50 nm GaAsP PMT, 600/50 nm GaAsP PMT and 685/70 nm PMT (A1-DUG-2, Nikon). The fluorescence mode can be combined with brightfield or DIC.
For laser scanning spectral imaging mode, the sample is excited with 405 nm, 488 nm, 561 nm, and 640 nm laser from the laser unit (LU-NV, Nikon). Galvano scanner can zoom and rotate scan direction, maximum pixels 2048 x 2048. Excitation light passes through an adjustable pinhole and is directed to the sample by a dichroic mirror 405/488/561/640 or an 80/20 beam splitter. A spectrum can be acquired from 400 – 750 nm and is divided in maximum 32 channels each with a bandwidth of 2.5, 6 nm or 10 nm. The signal is recorded by an array of 32 PMT detectors coinciding with the 32 channels (A1-DUS, Nikon).
For laser scanning imaging with tunable emission filters, as spectral imaging mode, but the array of 32 detectors is set up as virtual 4 detectors.
For SIM mode (N-SIM, Nikon), the sample is excited with 405 nm, 488 nm, 561 nm, and 640 nm laser from the laser unit (LU-NV, Nikon). For 2D and 3D SIM, the laser is mounted through a multi mode fiber. For these SIM techniques, all 4 lasers (405 nm, 488 nm, 561 nm and 640 nm) are compatible in combination with the objectives: Apo TIRF 60x 1.49, SR HP Plan Apo Lambda S 100x Sil NA 1.35, and SR HP Apo TIRF 100x NA 1.49. Each color channel has a single dichroic mirror and emission filter (blue channel: 453 – 485 nm, green channel: 500 – 545 nm, red channel: 570 – 640 nm, and far red channel: 663 – 738 nm). For TIRF-SIM, the laser is mounted through a single mode fiber and is only compatible with SR HP Apo TIRF 100x NA 1.49 in combination with the 488 nm and 561 nm lasers. The lasers are set at a fixed TIRF angle. Images are acquired with an iXon Ultra 897 EMCCD camera (Andor).
Location
Leichtag building, Room 471

